- All posts
- 8 Media Venture
- althetics
- aMMP8
- Annimari Korte
- Antibiotic resistance
- Apotek Härtat
- Aqua Dental
- athlete
- Award
- Baltics
- Bonnier
- Bonnier News
- Brain health
- Business
- Cancer
- cardiovascular disease
- caries
- Chemo therapy
- children
- collaboration agreement
- Croatia
- Denmark
- Denta
- Dental erosion
- Dentex
- diabetes
- Dual Light
- Duodecim
- EFP
- EFR
- Estonia
- EuroPerio
- event
- Expodental
- FIBO
- fund raising
- general health
- Gingivitis
- Gum disease
- HAP
- HealthHub Pharma
- HIDES
- Hospital infections
- hospital-acquired pneumonia
- IBD
- Iceland
- IDS COLOGNE
- implantology
- invest
- investment
- italy
- Koite Health
- Latvia
- Lithuania
- lumoral
- Lumoral App
- Lumoral Junior
- Maritime industry
- Media
- MegaGen
- Movie
- News
- Nordic markets
- Nordics
- O
- Olympics
- Oral health
- Oral hygiene
- Oral mucositis
- Patent
- PDT
- peri-implantitis
- Perio Master Clinic
- Periodontitis
- periodontology
- Photodynamic therapy
- Press
- Ranking
- Romania
- Scandinavian Society of Periodontology
- Science
- Seafarer
- Seedtable
- share issue
- Shareissue
- Siblings movie
- Spain
- spots
- Stroke
- Study
- sweden
- Tartar
- techtour
- Thailand
- UK
- United States
- Valentine's Day
- WHO
- World Cancer Day
- World Health Day
- World Heart Day
- world oral health day
- World Smile Day

The popularity of dental implants has increased globally, but ensuring their longevity requires meticulous and regular oral hygiene. This challenge has led to the introduction of a new Finnish inve...

Lumoral Showcased by Med-Faktor at Dentex 2024 Fair
The International Dental Medicine Fair, commonly known as Dentex, once again brought the dental industry to the forefront from June 6-8, 2024. Organized by Zagreb Fair and the Croatian Chamber of D...
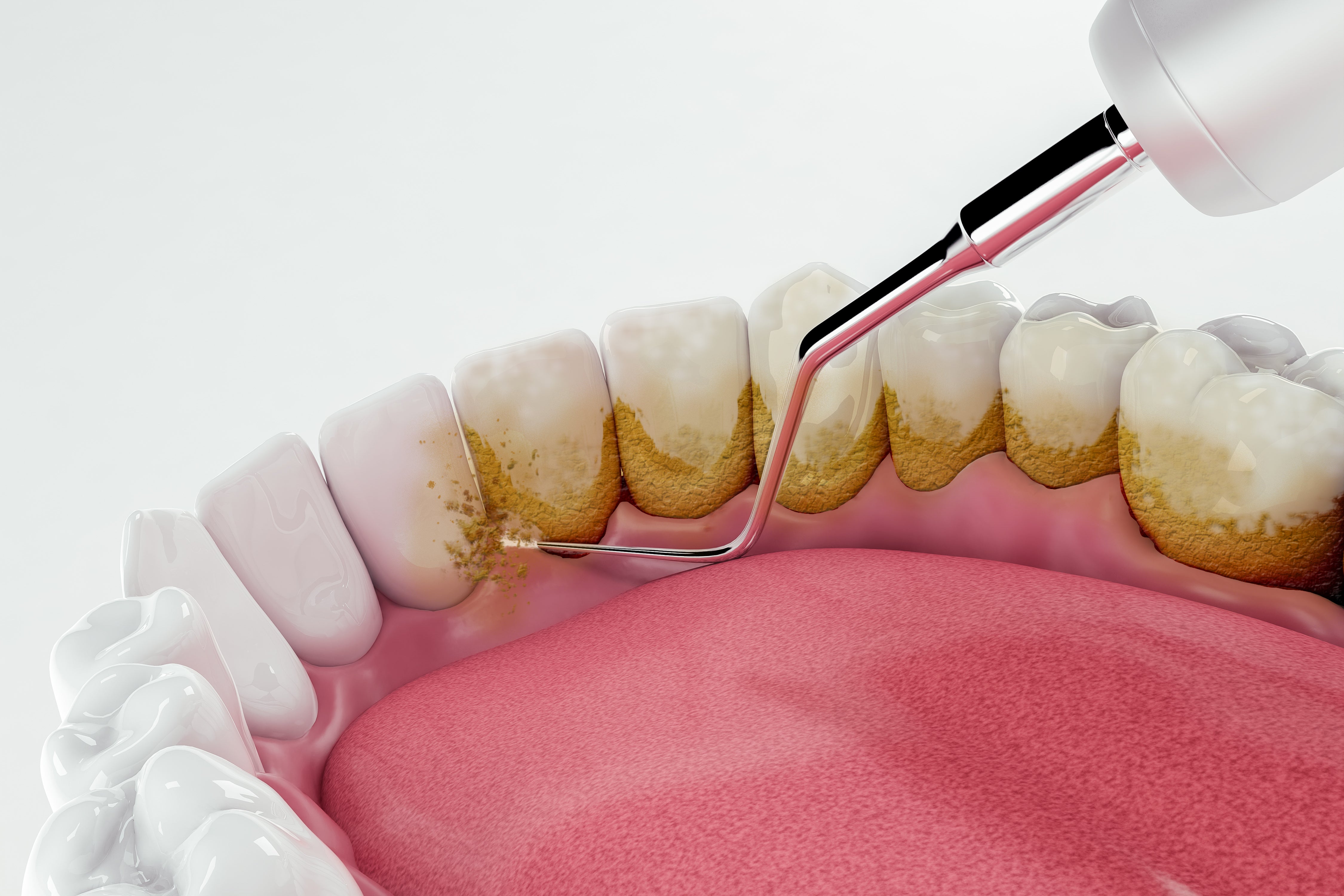
Taking control of troublesome tartar
Tartar is mineralised plaque that forms on the surface of the teeth, usually as a result of poor oral hygiene. Plaque is made up of bacteria, food debris and saliva proteins. If plaque is not regul...

17th MegaGen International Symposium Shines the Spotlight on Antibacterial Lumoral Treatment
The 17th MegaGen International Symposium in Rome, Italy, will be held from May 30 to June 1, 2024. Organized by MegaGen, a leading dental implant company, the event gathers dental experts worldwide...

Dental disease that many overlook – this is what you should check for
An untreated inflammation in the gums can eventually lead to periodontitis. Yet, many people overlook the symptoms of gingivitis, as it is also called. Here are the signs you should be on the looko...
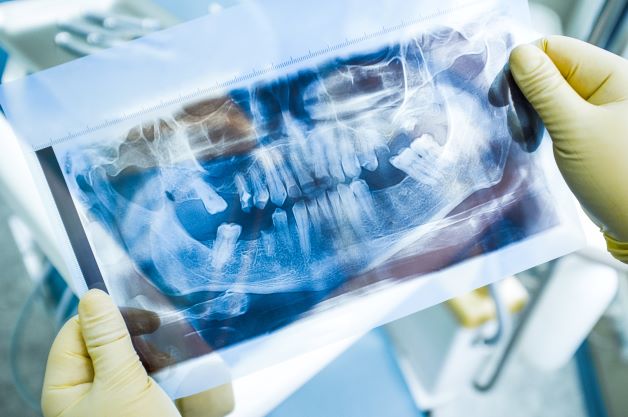
Treatment of Periodontitis in Smoking Patients with the Lumoral Method
(This article is based on the writing of dentist Viktoria Ljutkina, published in the largest dental medicine periodical in Estonia, Ajakiri Hambaarst. Ljutkina works as a dentist at Läänemere Hamba...