Koite Health Oy is a Finnish health technology company that develops light-activated antibacterial solutions for the treatment and prevention of oral diseases. The company’s Lumoral teeth cleaning method can be used at home by children and adults to prevent tooth decay and gum diseases and treat even the most severe cases of gum disease including periodontitis.
Mikko Kylmänen, Clinical Research Project Manager at Koite Health, explains the importance of high standard clinical research and the different stages that took place before the innovative Lumoral method for oral self-care became available to consumers.
Why is clinical research needed?
Clinical research is needed to ensure that healthcare devices on the market are safe to use, are fit for their intended purpose and meet the characteristics and performance standards required. When clinical trials are conducted to a high standard and based on scientific evaluation, both users and healthcare professionals can have confidence in the quality and performance of the medical device.
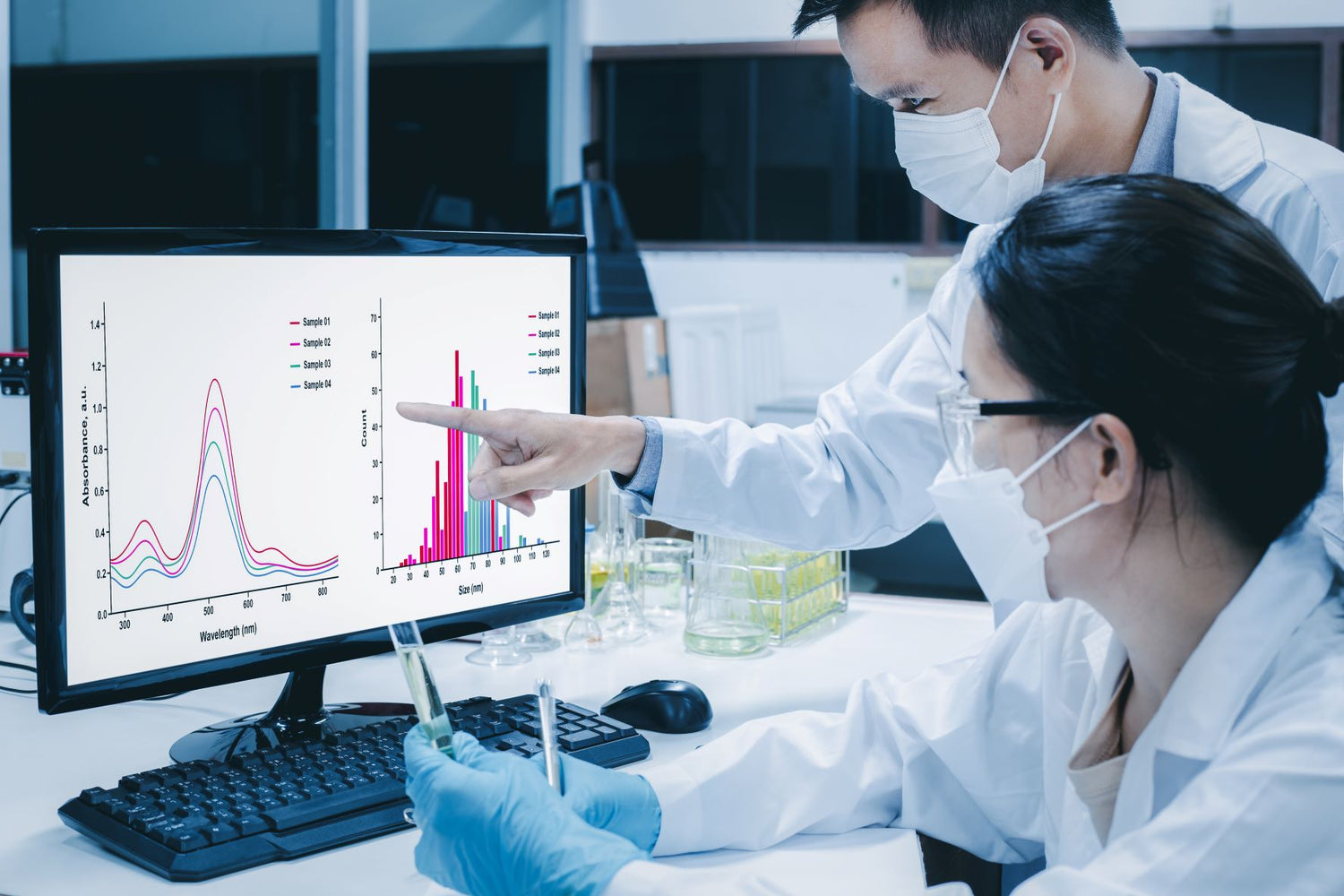
The development of medical technologies is based on multidisciplinary research, which continues actively even after the product has been launched on the market. The effect of regular use of Lumoral has been – and still is – the subject of multiple clinical trials, for example, in the treatment of dental implant-related infections. The results have been promising: plaque amount and gum bleeding around the inflamed gum tissues of patients were significantly reduced compared to the initial situation. More research on this topic is expected to be released later in 2023.
In December 2022, Koite Health published the interim report of a large periodontal study in an international dental journal.
– The findings were so significant that we wanted to bring them to the attention of the professional and scientific communities, and also Lumoral users as soon as possible. At the same time, seeing these results gave us at Koite Health more information about the performance and safety of the device. The new information also can be used when new studies are planned, Mikko Kylmänen concludes.
Standards are the basis for clinical research
According to Kylmänen, international standards are the cornerstone of clinical trial designs for Lumoral. These include ISO 14155 GCP (Clinical Trials on Medical Devices - Good Clinical Practice) and the European Union's Medical Device Regulation EU 2017/745 (MDR).
A Lumoral clinical trial must have clear goals and objectives, either to ensure the effects of the treatment as already authorised, or to find new uses for Lumoral. Strict compliance with regulations will ensure that the safety of the study subjects is not compromised.
– As a rule, our studies are randomised controlled trials between two study groups - the Lumoral group and the control group. Participants in both study groups will undergo exactly the same clinical measurements and will be given exactly the same self-care instructions. The only difference between the two groups is that one group uses Lumoral during the study and the other does not.
Randomisation means that the study group for a subject is randomly selected and neither the subject nor the researcher knows which group the subject belongs to before the study starts.
The company has sometimes been asked why it does not conduct placebo-controlled trials with Lumoral. According to Mr Kylmänen, it is quite common internationally that in device studies, subjects in one group have the device and not the other. Placebo-controlled studies are better suited to medical trials.
Brainstorming of studies is a collaborative process
The best ideas for studies often come from open discussion with professionals such as dentists, oral hygienists or researchers. These discussions gradually lead to the development of a research outline, which is then refined by researchers in collaboration with the professionals involved in the individual study.
According to Kylmänen, involving professionals in Lumoral studies at an early stage is crucial because they know best the needs of their own speciality.
– We always try to take into account the typical treatment frequency of the respective disease at the clinic. When planning this way, there is as little extra effort as possible for the study subjects.
Careful preparation is required to obtain authorisation for studies
All study plans for Lumoral studies – including the materials to be provided to subjects and the documentation related to data protection - are subject to an application to a research ethics committee for an opinion. As a rule, the committee is selected according to where the study will be conducted. Issues such as data protection are also carefully considered at this stage.
When the committee finally gives its opinion, it is the turn of the Finnish Medicines Agency (Fimea), or equivalent authority in other countries, to give approval to start the clinical study. Fimea's tasks include the supervision of medical devices in Finland under the MDR Regulation. Clinical trials of devices are also subject to supervision.
– If Fimea requires changes to the study plan, the changes must be resubmitted to the Ethics Committee, either as a new application or as a notification. Overall, the application process for regulatory approvals in Finland takes at least three months. Usually even longer. After that, approvals are still needed from the clinics where the research will be carried out.
Contracts are concluded according to need and local practice
Various contracts can be concluded with researchers and clinics, depending on need and local practice. Often a local research authorisation also requires a research contract to be drawn up.
– We also take care of the costs of the sponsored studies, and these are written into the contracts. We also have contracts with so-called CROs (contract research organisations), from whom we buy research monitoring services, says Kylmänen.
Good clinical practices guide Lumoral studies
One of the most important principles of clinical research is that no measurement or procedure related to the study should be performed on any patient without their written consent.
According to Kylmänen, good clinical practice in Lumoral trials also includes training and ensuring the competence of the research team and providing background support for researchers throughout the trial. The MDR regulation, on the other hand, requires close monitoring of the benefits and potential harms of the device.
– Of course, this is also in our interests from the point of view of the device developer, Kylmänen stresses.
Quality control is an important part of clinical research
For each trial, an individual quality control or monitoring plan is drawn up. This covers among other things, the consent collection process, and recording and reporting of adverse events. It also covers the review of trial data before the trial results are analyzed.
Monitoring is one of the most important steps in the overall safety and scientific credibility of a clinical trial, according to Kylmänen.
Clinical research is like a marathon
Kylmänen points out that science is complex. Even if "your own thing" may seem clear to you, you may have to explain it in great detail to other scientists – even co-scientists.
– Even the most thorough explanation does not necessarily guarantee that everyone will believe you. Research results come slowly. If a study starts today, it could be two or more years before it reaches the publication stage. In fact, I now think of a clinical trial as more than just a single marathon. It is a series of marathons. Like running in slightly different landscapes repeatedly. And even if you reach one goal, there may be several journeys yet to be completed and new ones to meet.
– However, I must add that the launch of Lumoral to the market did not happen by chance. The idea to develop an antibacterial teeth cleaning Lumoral method was based on scientific evidence from the outset. It took three years from the initial concept to the launch of the current product. Now the final product is unlike anything we have ever seen before. Helping to bring clinic-level treatment to a relatively hassle-free home-based treatment. Without compromising on quality.

Leave a comment
This site is protected by hCaptcha and the hCaptcha Privacy Policy and Terms of Service apply.